The 3rd MPRC Competition
Topic of the 3rd period of MPRC competitions was Mn, the #4 most commonly used metal globally. The challenge was the suggestion of a process to leach of this element with the highest efficiency and selectivity against Fe. The material considered for this purpose was milled pyrolusite (MnO4) ore containing 22% Mn.
For the third time, the three of us started to review scholarly articles about the topic. In conclusion, we decided to use the Glycine aqueous solution as a solvent and the Ascorbic Acid as a reducing agent. We chose this solvent because good results had been obtained at using these agents in the case of battery recycling by prior researchers. By the way, this new process would cover the innovative aspect needed.
Accordingly, we proposed a simple process showed below:
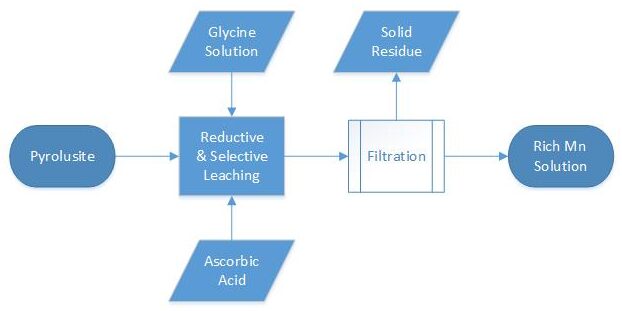
At this easy process, that is performed in 85 degrees centigrade, reactions of reduction and complexation or solvation have been reported as below, respectively:
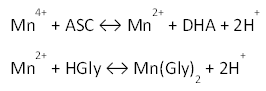
Following figures are rich Mn solution and solid residue obtained during our suggested process and delivered to evaluation. Totally, referees reviewed competitors’ performance based on following criteria:
- Selectivity ([Mn]/[Fe]),
- Oral and written presentation,
- Executive quality,
- Creativity.

This was my last presence in these competitions and we succeeded in achieving the 2nd best team award. I’m so grateful to my teammates that accompanied me in this fantastic journey, Engineers Karimi and Farahani.

Leave a Reply