Bachelor’s Thesis
Why materials science and engineering?
As I mentioned in the above post about my passion for chemistry, I found the field of extraction of metals more interesting in comparison with other branches of metallurgy. Accordingly, for my BSc thesis, I try to select an available subject related to this area. In this regard, I’ve researched zinc and cadmium separation through solvent extraction method. This research was part of a project in which the effect of different parameters, especially synergistic and complexation, on the separation of Zn, Cd, Mn, Ni, Co, and Cu from sulfate leach liquor of e-waste recycling was investigated.
In the case of synergistic effect, mixture of D2EHPA with TBP, CYANEX® 272, and CYANEX ®302 had been investigated. Considering the complexation effect, presence of Tartrate[1], Acetate[2], and Citrate[3] ions had been evaluated in the Ni-Co system previously. After that, another graduate student and I studied the effect of, respectively, Tartrate and Citrate ions on Zn and Cd separation.
To this purpose, solution of %20 v/v D2EHPA in Kerosene was used as organic solvent, and pure sulfate compounds of zinc and cadmium were purchased to make a stock aqueous solution. About 30 experiments in different concentrations of citrate ion (0.05 M – 0.15 M) and pH (0-4) were designed and performed at room temperature and [Zn2+]=[Cd2+]=5 g/l. To determine the concentration of Zn and Cd ions, AAS technique was employed.
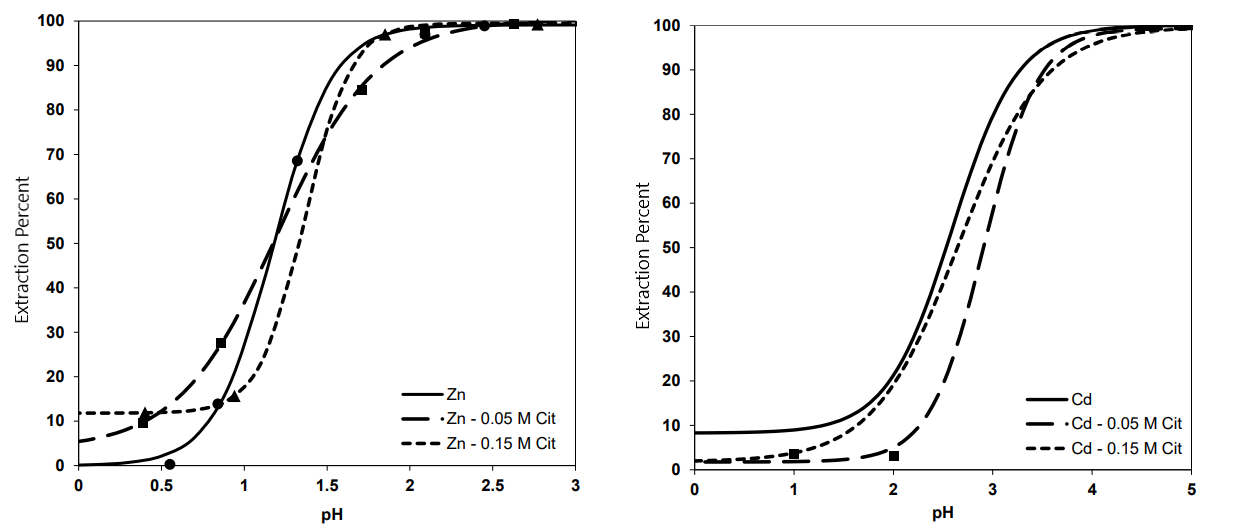
We found that citrate ion causes changes in extraction percent diagrams of Zn and Cd as can be seen below:
According to the results, adding 0.05 M citrate ion to the system enhances the separation due to rising the ΔpH0.5 from 1.32 to 1.76, while addition of 0.15 M citrate ion does not change this factor. Moreover, the amount of separation, enrichment, and ΔE factors are maximum in this concentration at pHs of 2.9, 1.5, and 2.1, respectively.
Furthermore, provided data were analyzed and in summary, following reactions were suggested as extraction mechanisms of zinc and cadmium in 0.05 M citrate ion and mentioned circumstances:
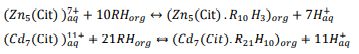
Unfortunately, some changes occurred in the AAS analyzing system during my work, including its operator. Also, obtained data according to delivered analyses were odd in comparison with previous investigation. Due to these two points, it was necessary to repeat experiments to be sure about the result and then be able to publish them, that time and cost were obstacles to do so.
At this research, professors Haghshenas and Firoozi kindly conducted me as my supervisors and I appreciate it. Also, I would like to thank Eng. Bahrami and Prof. Azadmehr, who helped me to solve some problems, as well as, Dr. Nadimi and Ms. Vahedi, BSc, who taught me based on their previous experiences in this project.

Leave a Reply